| In Brief |
|
Technological advancements in the electric battery field are generating significant interest, particularly due to their potential to transform how we consume energy. Recently, a team of scientists from Dongguk University in Seoul, South Korea, announced a significant breakthrough in lithium-ion battery technology. This innovation promises to reduce the size and weight of energy storage devices while enhancing their efficiency. With a revolutionary hybrid anode material, this discovery could disrupt the electric vehicle industry and beyond by dramatically improving storage capacity and long-term stability during charge cycles. Let’s explore the details of this promising advancement.
High Conductivity and Large Energy Storage Capacity
The new material developed by the research team combines the high conductivity of graphene oxide with the energy storage capacity of nickel-iron compounds. This combination could have a major impact on various industries, including electric vehicles, consumer electronics, and renewable energy. Under the supervision of Professor Jae-Min Oh, researchers utilized nano-engineered materials to design new energy storage solutions.
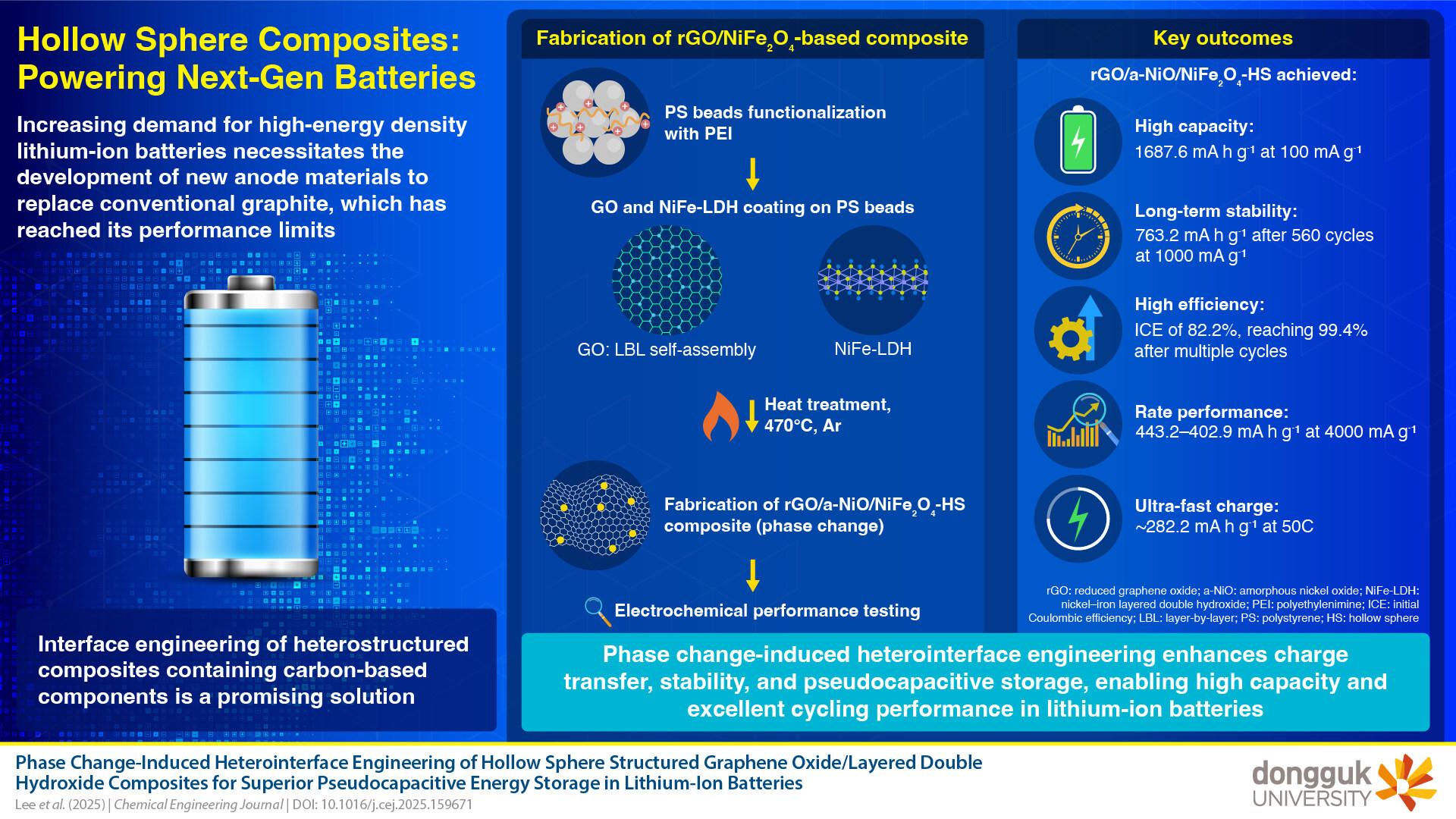
As described in the press release from Dongguk University, the study, published in the Chemical Engineering Journal, focuses on an innovative material designed to maximize the synergistic effects of its components. The composite is a hierarchical heterostructure that combines reduced graphene oxide (rGO) with nickel-iron layered double hydroxides (NiFe-LDH). The rGO provides a conductive network for electron transport, while the nickel-iron-based components enable rapid charge storage through a pseudocapacitive mechanism. The secret to this innovative design lies in the abundance of grain boundaries, facilitating efficient charge storage.
Smaller, Lighter, and More Efficient
The researchers used a “layer-by-layer self-assembly” technique to develop their final composite. This method involved coating polystyrene (PS) spheres with precursors of GO and NiFe-LDH. After removing the templates, a hollow spherical architecture was obtained. A controlled thermal treatment then induced a phase transformation in the NiFe-LDH, leading to the formation of nanocrystalline nickel-iron oxide (NiFe2O4) and amorphous nickel oxide (a-NiO), while simultaneously reducing the GO to rGO.
The scientists analyzed their composite using X-ray diffraction and transmission electronic microscopy. These analyses revealed promising results. Electrochemical tests also demonstrated the exceptional performance of the material as a lithium-ion battery anode. During these tests, the anode exhibited a high specific capacity of 1687.6 mA h g-1 at a current density of 100 mA g-1 after 580 cycles. According to the team, this significantly surpasses conventional materials used in traditional batteries.
Real-World Application Prospects
The real-world application prospects of this technology are vast. The researchers estimate that their development could be integrated into electronic devices within the next 5 to 10 years. This advancement paves the way for smaller, lighter, and more efficient energy storage devices suited for next-generation electronics. Professor Jae-Min Oh explained that future energy storage materials will exceed the improvement of individual components to involve multiple interactive materials creating synergy, resulting in more efficient and reliable energy storage devices.
While the team’s research shows great potential, further testing is required before this composite can truly transform our daily lives. However, the implications of this research could lead to more sustainable batteries that charge faster and are lighter in the future.
The Challenge of Commercialization
One of the major challenges for these researchers is to transition from theory to practice by integrating their discovery into commercial applications. This will require collaboration with the industry to adapt their innovations to existing manufacturing processes. Additionally, economic considerations, such as material costs and the feasibility of large-scale production methods, will play a crucial role in this transition.
Furthermore, the environmental impact of these new materials will need to be assessed to ensure that the advancements made do not come at the expense of our planet. Commitment to sustainable practices is essential to ensure that these innovations benefit society as a whole. The question remains: how will these technological advancements be incorporated into our daily lives to maximize their benefits while minimizing their ecological footprint?